Abstract
Background
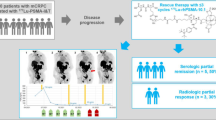
In recent years, theranostics has become a promising approach for treating metastatic castration-resistant prostate cancer (mCRPC), with trials investigating targeted radioligand therapy, particularly using prostate-specific membrane antigen labeled with lutetium-177 ([177Lu]Lu-PSMA). The proper position of [177Lu]Lu-PSMA in the therapeutic algorithm of mCRPC is yet to be identified.
Design, Setting, and Participants
We conducted a systematic review and meta-analysis of phase II/III randomized controlled trials to assess the efficacy of [177Lu]Lu-PSMA in treating mCRPC. Study endpoints included radiographic progression-free survival (rPFS), prostate-specific antigen-PFS, objective response rate, and overall survival.
Outcome Measurements and Statistical Analysis
Data were extracted according to the PRISMA statement. Summary hazard ratios (HRs) were calculated using random- or fixed-effects models. Statistical analyses were performed with RevMan software (v.5.2.3).
Results
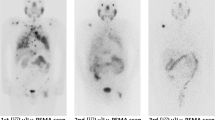
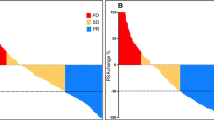
[177Lu]Lu-PSMA reduced the risk of rPFS (HR 0.55; 95% confidence interval [CI] 0.43–0.71; p < 0.00001) and prostate-specific antigen-PFS (HR 0.53; 95% CI 0.41–0.67; p < 0.00001), and improved the objective response rate compared with control therapies (response rate 3.55; 95% CI 1.91–6.60; p < 0.0001), whereas no significant cumulative effect on overall survival was documented (HR 0.92; 95% CI 0.65–1.31; p = 0.63). Notably, in a dedicated subanalysis, comparable effects on rPFS were observed when [177Lu]Lu-PSMA was compared with active therapy.
Conclusion
[177Lu]Lu-PSMA has a favorable impact on the radiographic and biochemical control of mCRPC and represents a potential treatment in a scenario where other valuable options are available. Further efforts are required to identify clinical and molecular markers necessary for proper patient stratification.





Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Bauckneht M, Ciccarese C, Laudicella R, et al. Theranostics revolution in prostate cancer: basics, clinical applications, open issues and future perspectives. Cancer Treat Rev. 2024;124: 102698. https://doi.org/10.1016/j.ctrv.2024.102698.
Hofman MS, Violet J, Hicks RJ, et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet. 2018;19(6):825–33. https://doi.org/10.1016/S1470-2045(18)30198-0.
Sartor O, De Bono J, Chi KN, et al. Lutetium-177–PSMA-617 for metastatic castration-resistant prostate cancer. N Eng J Med. 2021;385:1091–103. https://doi.org/10.1056/NEJMoa2107322.
Sartor O, Castellano Gauna DE, Herrmann K, et al. Phase III trial of [177Lu]Lu-PSMA-617 in taxane-naive patients with metastatic castration-resistant prostate cancer (PSMAfore). Ann Oncol. 2023;34(suppl_2):S1281–2. https://doi.org/10.1016/S0923-7534(23)04149-2.
Sartor O, Jiang DM, Smoragiewicz M, et al. Efficacy of 177Lu-PNT2002 in PSMA-positive mCRPC following progression on an androgen-receptor pathway inhibitor (ARPI) (SPLASH). Ann Oncol. 2024;35(suppl_2):1–72. https://doi.org/10.1016/annonc/annonc1623.
Hofman MS, Emmett L, Sandhu S, et al. 177Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet. 2021;397(10276):797–804. https://doi.org/10.1016/S0140-6736(21)00237-3.
Hofman MS, Emmett L, Sandhu S, et al. Overall survival with [177Lu]Lu-PSMA-617 versus cabazitaxel in metastatic castration-resistant prostate cancer (TheraP): secondary outcomes of a randomised, open-label, phase 2 trial. Lancet. 2024;25(1):99–107. https://doi.org/10.1016/S1470-2045(23)00529-6.
Rosar F, Dewes S, Ries M, et al. New insights in the paradigm of upregulation of tumoral PSMA expression by androgen receptor blockade: enzalutamide induces PSMA upregulation in castration-resistant prostate cancer even in patients having previously progressed on enzalutamide. Eur J Nucl Med Mol Imaging. 2020;47:687–94. https://doi.org/10.1007/s00259-019-04674-0.
Emmett L, Subramaniam S, Crumbaker M, et al. [177Lu]Lu-PSMA-617 plus enzalutamide in patients with metastatic castration-resistant prostate cancer (ENZA-p): an open-label, multicentre, randomised, phase 2 trial. Lancet Oncol. 2024;25(5):563–71. https://doi.org/10.1016/S1470-2045(24)00135-9.
Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. https://doi.org/10.1016/0197-2456(95)00134-4.
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47. https://doi.org/10.1016/j.ejca.2008.10.026.
Scher HI, Morris MJ, Stadler WM, et al. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the prostate cancer clinical trials working Group 3. J Clin Oncol. 2016;34(12):1402–18. https://doi.org/10.1200/JCO.2015.64.2702.
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. https://doi.org/10.1136/bmj.n71.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. https://doi.org/10.1136/bmj.327.7414.557.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. https://doi.org/10.1016/0197-2456(86)90046-2.
Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration. 2014. http://ims.cochrane.org/revman/download.
Iacovelli R, Ciccarese C, Schinzari G, et al. Going towards a precise definition of the therapeutic management of de-novo metastatic castration sensitiveprostate cancer patients: how prognostic classification impact treatment decisions. Crit Rev Oncol Hematol. 2019;139:83–6. https://doi.org/10.1016/j.critrevonc.2019.05.005.
Ciccarese C, Iacovelli R, Sternberg CN, Gillessen S, Tortora G, Fizazi K. Triplet therapy with androgen deprivation, docetaxel, and androgen receptor signalling inhibitors in metastatic castration-sensitiveprostate cancer: a meta-analysis. Eur J Cancer. 2022;173:276–84. https://doi.org/10.1016/j.ejca.2022.07.011.
Fizazi K, Gillessen S, ESMO Guidelines Committee. Updated treatment recommendations for prostate cancer from the ESMO Clinical Practice Guideline considering treatment intensification and use of novel systemic agents. Ann Oncol. 2023;34(6):557–63. https://doi.org/10.1016/j.annonc.2023.02.015.
Flippot R, Telli T, Velev M, et al. Activity of Lutetium-177 prostate-specific membrane antigen and determinants of outcomes in patients with metastatic castration-resistant prostate cancer previously treated with cabazitaxel: the PACAP study. Eur Urol Oncol. 2024;7(5):1132–40. https://doi.org/10.1016/j.euo.2024.03.013.
De Wit R, De Bono J, Cora N, et al. Cabazitaxel versus abiraterone or enzalutamide in metastatic prostate cancer. N Engl J Med. 2019;381:2506–18. https://doi.org/10.1056/NEJMoa1911206.
Fizazi K, Piulats JM, Reaume MN, et al. Rucaparib or physician’s choice in metastatic prostate cancer. N Engl J Med. 2023;388(8):719–32. https://doi.org/10.1056/NEJMoa2214676.
Hussain M, Mateo J, Fizazi K, et al. Survival with olaparib in metastatic castration-resistant prostate cancer. N Engl J Med. 2020;383(24):2345–57. https://doi.org/10.1056/NEJMoa2022485.
Van Wambeke S, Vera-Badillo FE, Gyawali B. Controlling the control arm in metastatic castration-resistant prostate cancer trials: best standard of care or the minimum standard of care? J Clin Oncol. 2022;40(14):1518–21. https://doi.org/10.1200/JCO.21.02304.
Maines F, Caffo O, Veccia A, et al. Sequencing new agents after docetaxel in patients with metastatic castration-resistant prostate cancer. Crit Rev Oncol Hematol. 2015;96(3):498–506. https://doi.org/10.1016/j.critrevonc.2015.07.013.
Azad AA, Bressel M, Tan H, et al. Sequential [177Lu]Lu-PSMA-617 and docetaxel versus docetaxel in patients with metastatic hormone-sensitive prostate cancer (UpFrontPSMA): a multicentre, open-label, randomised, phase 2 study. Lancet Oncol. 2024;25(10):1267–76. https://doi.org/10.1016/S1470-2045(24)00440-6.
Seifert R, Telli T, Lapa C, et al. Safety and efficacy of extended therapy with [177Lu]Lu-PSMA: a German Multicenter Study. J Nucl Med. 2024;65(6):909–16. https://doi.org/10.2967/jnumed.123.267321.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funding was used in the preparation of this manuscript.
Conflict of interest
Chiara Ciccarese has received speaker honoraria from Astellas, BMS, EISAI, IPSEN, Janssen, MSD, Novartis, Pfizer, and Sanofi. Matteo Bauckneht has received speaker honoraria from Novartis and GE Healthcare. Giampaolo Tortora is an advisory board member for BMS and Novartis. Luca Zagaria has received speaker honoraria from Novartis. Orazio Caffo has received speaker honoraria from Astellas, Astra Zeneca, Bayer, Janssen, Ipsen, MSD, Novartis, Pfizer, and Recordati. Roberto Iacovelli is an advisory board member for Astellas, BMS, EISAI, IPSEN, Janssen, MSD, Novartis, Pfizer, and Sanofi and a consultant for Astellas, EISAI, MSD, and Pfizer. Giuseppe Fornarini, Viria Beccia, Francesco Lanfranchi, Germano Perotti, Giada Pinterpe, Fortuna Migliaccio, Lucia Leccisotti, Gianmario Sambuceti, and Alessandro Giordano declare that they have no conflicts of interest that might be relevant to the contents of this manuscript.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Code availability
Not applicable.
Authors' contributions
Conceptualization: C.C., M.B., and R.I. Data curation: L.Z., G.F., V.B., F.L., G.P., G.P., F.M., G.T., L.L., G.S., A.G., and O.C. Formal analysis: C.C., M.B., and R.I. Investigation: C.C., and M.B. Methodology: all authors. Software: C.C. and R.I. Writing - original draft: C.C., M.B., and R.I. Writing - review & editing: all authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ciccarese, C., Bauckneht, M., Zagaria, L. et al. Defining the Position of [177Lu]Lu-PSMA Radioligand Therapy in the Treatment Landscape of Metastatic Castration-Resistant Prostate Cancer: A Meta-analysis of Clinical Trials. Targ Oncol 20, 103–112 (2025). https://doi.org/10.1007/s11523-024-01117-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-024-01117-1